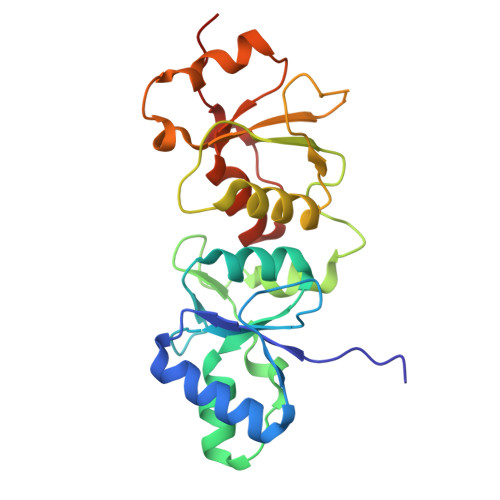
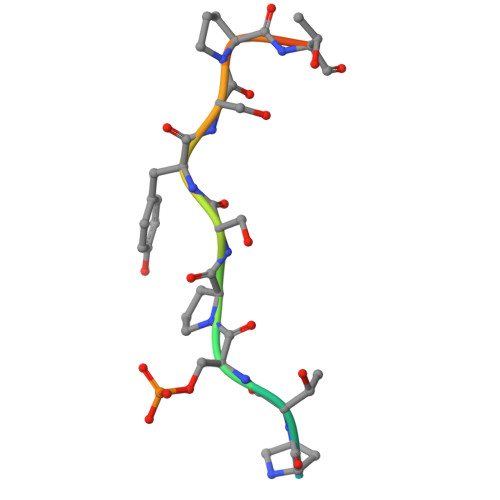
Distinct mechanisms of recognition of phosphorylated RNAPII C-terminal domain by BRCT repeats of the BRCA1-BARD1 complex.
Klapstova, V., Sedova, K., Houser, J., Sebesta, M.(2025) J Biological Chem 302: 111010-111010
- PubMed: 41354346
- DOI: https://doi.org/10.1016/j.jbc.2025.111010
- Primary Citation of Related Structures:
9QPX - PubMed Abstract:
Transcription competes with other DNA-dependent processes, such as DNA repair, for access to its substrate, DNA. However, the principles governing the interplay between these processes remain poorly understood. Evidence suggests that the BRCA1-BARD1 complex, a key player in the DNA damage response, may act as a mediator of this crosstalk. In this study, we investigated the molecular mechanism underpinning the interaction between RNA polymerase II (RNAPII) and the BRCA1-BARD1 complex, as well as its functional implications. Our findings reveal that the tandem BRCT domain of BRCA1 binds the Ser5-phosphorylated CTD of RNAPII, utilizing a mechanism previously established for other BRCA1 BRCT ligands. Furthermore, we demonstrate that this interaction is critical for the organization of RNAPII into condensates with liquid-like properties. Analysis of disease-associated variants within the BRCT repeats further supports the biological relevance of this condensation. Collectively, our results suggest that the BRCA1-BARD1 complex may coordinate transcription and DNA repair by facilitating the organization of RNAPII into transcription factories.
- CEITEC-Central European Institute of Technology, Masaryk University Brno, Czechia; National Centre for Biomolecular Research, Faculty of Science, Masaryk University Brno, Czechia.
Organizational Affiliation: