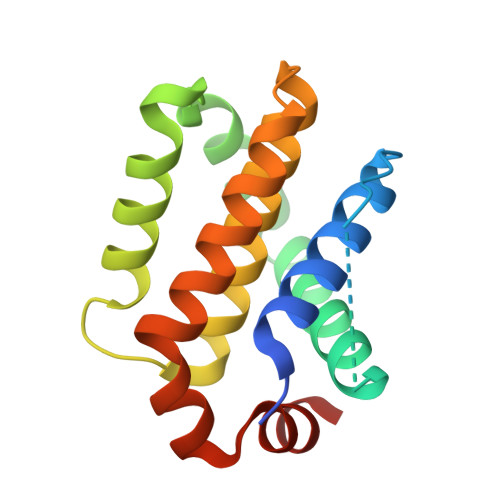
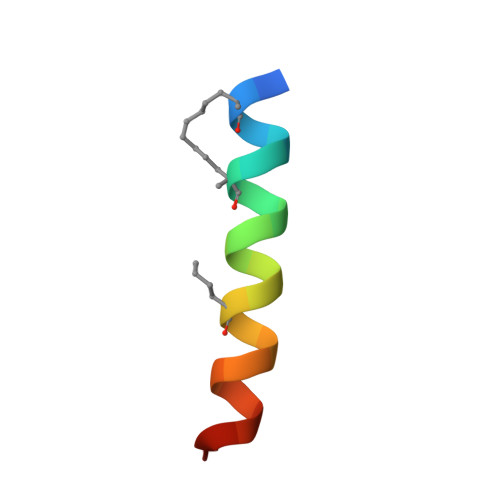
Structural insights into chemoresistance mutants of BCL-2 and their targeting by stapled BAD BH3 helices.
DeAngelo, T.M., Adhikary, U., Korshavn, K.J., Seo, H.S., Brotzen-Smith, C.R., Camara, C.M., Dhe-Paganon, S., Bird, G.H., Wales, T.E., Walensky, L.D.(2025) Nat Commun 16: 8623-8623
- PubMed: 41022713
- DOI: https://doi.org/10.1038/s41467-025-63657-y
- Primary Citation of Related Structures:
9O14, 9O15, 9O16 - PubMed Abstract:
BCL-2 is a central regulator of apoptosis and inhibits cell death by sequestering pro-apoptotic BH3 alpha-helices within a hydrophobic surface groove. While venetoclax, a BH3-mimetic drug, has transformed the treatment of BCL-2-driven malignancies, its efficacy is increasingly limited by acquired resistance mutations that disrupt small-molecule binding yet preserve anti-apoptotic function-reflecting a remarkable structural adaptation. Here, we employ hydrocarbon-stapled alpha-helices derived from the BAD BH3 motif as conformation-sensitive molecular probes to investigate this therapeutic challenge. The stapled peptides not only retain high-affinity binding to all BCL-2 variants but also show enhanced potency to select venetoclax-resistant mutants. Structural analyses, including X-ray crystallography and hydrogen-deuterium exchange mass spectrometry (HDX MS), demonstrate that these stapled helices restore native BH3 engagement by reversing the conformational consequences of resistance mutations. Notably, we identify a serendipitous interaction between the α3-α4 region of BCL-2 and hydrocarbon staple, which further compensates for altered groove conformation and contributes to mutant binding affinity. Together, these findings offer mechanistic insights into BCL-2 drug resistance and reveal a blueprint for designing next-generation inhibitors that overcome this clinically significant barrier to durable treatment responses.
- Department of Pediatric Oncology, Dana-Farber Cancer Institute, Boston, MA, USA.
Organizational Affiliation: