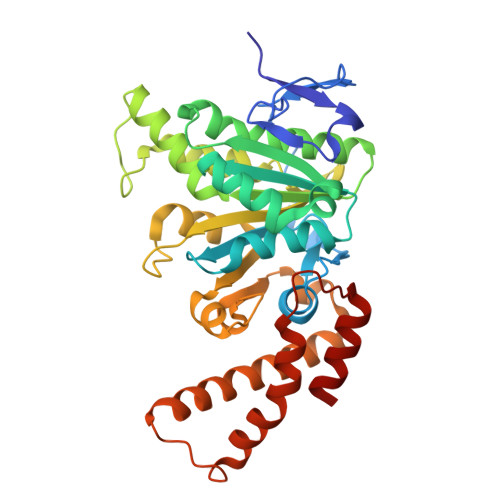
Structural and Biophysical Characterization of the Yersinia Type Three Secretion System ATPase YscN.
Barker, S.A., Ellis, P.K., Hammer, A., Johnson, S.J., Dickenson, N.E.(2026) Proteins
- PubMed: 41556233
- DOI: https://doi.org/10.1002/prot.70112
- Primary Citation of Related Structures:
9DMD, 9E58 - PubMed Abstract:
Yersinia pestis was responsible for the Black Plague, one of the worst epidemiological disasters in recorded history. Today, Y. pestis, Y. enterocolitica, and Y. pseudotuberculosis remain clinically relevant human pathogens. Each of these pathogenic Yersinia species relies on a Type Three Secretion System (T3SS) for virulence, with the ATPase YscN playing a critical role in T3SS function. T3SS ATPases are responsible for powering apparatus formation and effector protein secretion through ATP hydrolysis. This study provides an extensive enzymatic characterization of recombinant YscN under several conditions, including variable pH and temperature, substrate and protein concentrations, and in the presence of putative inhibitors. Thermal stability data, assessed by circular dichroism, demonstrate that YscN exhibits increased stability in alkaline conditions, coinciding with greatest ATPase activity. Further, we report the first high-resolution crystal structure of YscN and leverage homology data to model an oligomeric active site. Mutational analysis of a predicted active site residue confirms oligomerization as necessary for YscN ATPase activity and corroborates our oligomeric model and enzyme concentration-dependent specific activity. Interestingly, however, AUC analysis reveals that the purified YscN predominantly exists as a monomer, despite oligomerization-dependent active site formation. Thus, we propose that transient oligomeric interactions support the observed ATP hydrolysis. Together, these data uncover structural and environmental impacts on YscN activity that may support the highly specialized Yersinia pathogenic lifecycle and leverage its role in virulence in search of pan-effective small molecule T3SS ATPase inhibitors.
- Department of Chemistry and Biochemistry, Utah State University, Logan, Utah, USA.
Organizational Affiliation: