Fragment Screening and Structure-Guided Development of Heparanase Inhibitors Reveal Orthosteric and Allosteric Inhibition
Davies, L.J., Whitefield, C., Kim, H., Nitsche, C., Jackson, C.J., Frkic, R.L.(2026) ACS Med Chem Lett
Experimental Data Snapshot
Starting Model: experimental
View more details
(2026) ACS Med Chem Lett
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Heparanase 50 kDa subunit | 384 | Homo sapiens | Mutation(s): 0 Gene Names: HPSE, HEP, HPA, HPA1, HPR1, HPSE1, HSE1 EC: 3.2.1.166 | 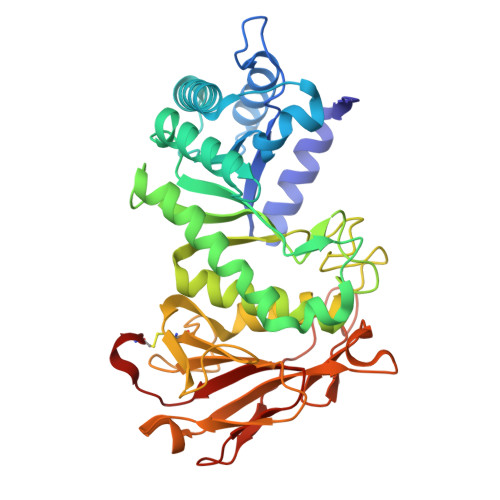 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q9Y251 (Homo sapiens) Explore Q9Y251 Go to UniProtKB: Q9Y251 | |||||
PHAROS: Q9Y251 GTEx: ENSG00000173083 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9Y251 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Heparanase 8 kDa subunit | 74 | Homo sapiens | Mutation(s): 0 Gene Names: HPSE, HEP, HPA, HPA1, HPR1, HPSE1, HSE1 EC: 3.2.1.166 | 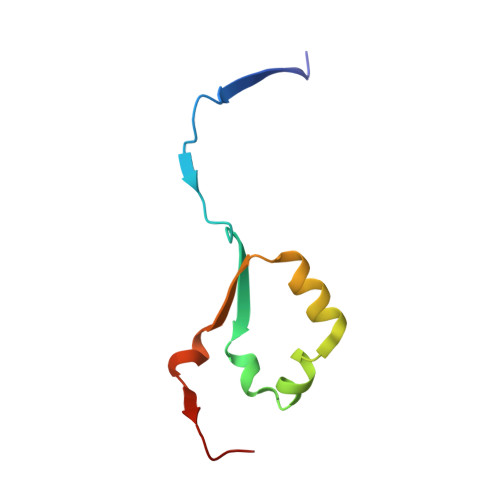 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q9Y251 (Homo sapiens) Explore Q9Y251 Go to UniProtKB: Q9Y251 | |||||
PHAROS: Q9Y251 GTEx: ENSG00000173083 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9Y251 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| LNR (Subject of Investigation/LOI) Query on LNR | J [auth B] | L-NOREPINEPHRINE C8 H11 N O3 SFLSHLFXELFNJZ-QMMMGPOBSA-N |  | ||
| PEG Query on PEG | C [auth A], D [auth A] | DI(HYDROXYETHYL)ETHER C4 H10 O3 MTHSVFCYNBDYFN-UHFFFAOYSA-N |  | ||
| SO4 Query on SO4 | E [auth A], F [auth A], G [auth A], H [auth A], I [auth A] | SULFATE ION O4 S QAOWNCQODCNURD-UHFFFAOYSA-L |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 58.581 | α = 90 |
| b = 74.204 | β = 90 |
| c = 123.402 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| Aimless | data scaling |
| XDS | data reduction |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Australian Research Council (ARC) | Australia | CE200100012 |
| Australian Research Council (ARC) | Australia | CE200100029 |