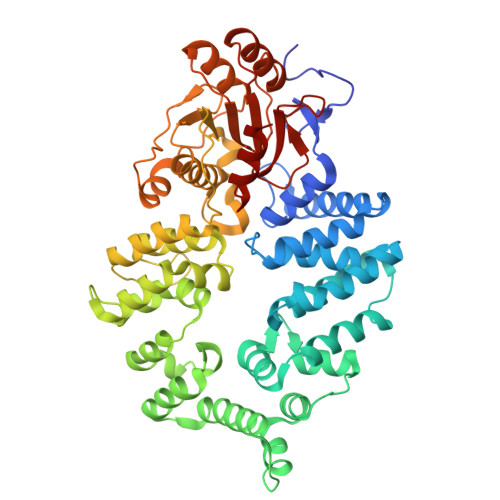
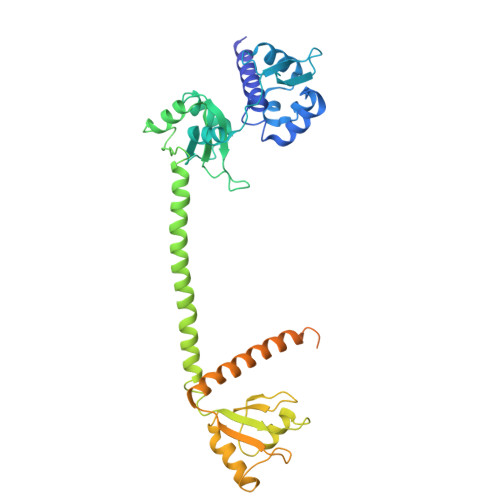
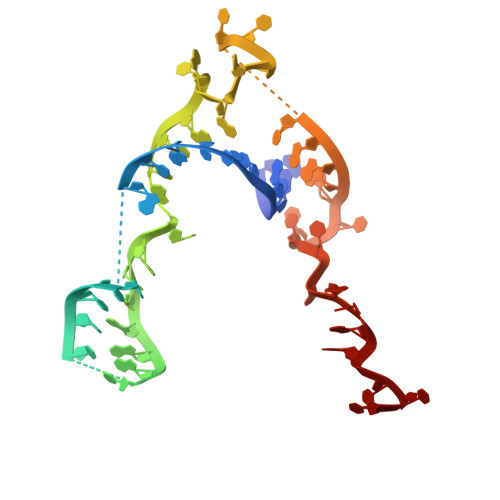
Mechanistic insights into RNA chaperoning by Ro60 and La autoantigens.
Nam, H., Deme, J.C., Sim, S., Boccitto, M., Lea, S.M., Wolin, S.L.(2026) Cell 189: 1135
- PubMed: 41610850
- DOI: https://doi.org/10.1016/j.cell.2025.12.030
- Primary Citation of Related Structures:
9NEN, 9NEP, 9NF8, 9NFA - PubMed Abstract:
Although ATP-independent chaperones assist RNA folding, the mechanisms by which they function remain elusive. Here, we demonstrate how two RNA chaperones collaborate to unfold misfolded noncoding RNAs (ncRNAs). The ring-shaped Ro60 protein binds the ends of misfolded ncRNAs in its cavity, whereas La stabilizes nascent ncRNAs and assists their folding. Using cryo-electron microscopy to resolve the structure of a misfolded RNA complexed with Ro60 and La, we show that La cradles the Ro60 ribonucleoprotein (RNP), with its N-terminal domain binding the RNA 3' end after it passes through the Ro60 cavity, while its C-terminal domain destabilizes structures in the misfolded RNA body. Using selective 2'-hydroxyl acylation analyzed by primer extension and mutational profiling (SHAPE-MaP), we show that La and Ro60 function synergistically to unfold non-native structures. As the RNAs bound by Ro60 and La include both ncRNA precursors and ncRNAs with oligouridine tails, this RNA chaperone machine may function widely to recognize misfolded and otherwise aberrant ncRNAs and assist their unfolding.
- RNA Biology Laboratory, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Frederick, MD 21702, USA.
Organizational Affiliation: